How Tea is Decaffeinated: Expunging the jingle-jangle: Part 3
Ah, the web. Look up ‘how to decaffeinate tea” and you will find breathless missives advocating infusing tea for 30 seconds or up to five minutes as the cheap, foolproof way to extract caffeine from tea. Nice myth. Or as tea expert /educator Bruce Richardson, owner of Elwood Teas, once wrote, “It was too good to be true.” (Based in Danville, Kentucky, Elmwood Teas also has a good selection of decaf teas.) So how is tea  transformed from caff to decaf?
transformed from caff to decaf?
There are two methods. One uses ethyl acetate, the other “super-critical” carbon dioxide. If you turn up your retrousse nose at the idea of a chemical polluting your cup, you’re only half right. Here’s why: Ethyl acetate is an organic compound that occurs naturally in tea. (That’s why it is often called “naturally” decaffeinated.) To decaffeinate tea using this method, leaves are moistened or soaked in water and then exposed to ethyl acetate, which is a solvent. Caffeine is then dissolved out of the leaves.The problem with this is that a lot of catechins, the anti-oxidents that carry much of tea’s health benefits, also leach out. As much 30% of Epigallocahtchin gallate (EGCG), is lost using this method. LTR readers know that EGCG has been touted as the substance in tea that reduces risks for heart disease, lowers blood pressure, even promotes bone health. (see previous posts.)
The other method uses “super-critical carbon dioxide,” or highly pressurized carbon dioxide. Carbon dioxide (CO2) is a gas under normal pressure and temperature. When pressure and temperature are increased, it acts midway between a gas and a liquid. This “supercritical CO2” is pumped into a sealed container with the tea, then circulated to remove the caffeine. The CO2 is then separated from the tea and pumped into a washer where water and/or charcoal separates it from the caffeine. The purified carbon dioxide then goes back into the pressurized chamber and the process is repeated to remove the designated amount of caffeine. (Thanks to Specialty Tea Institute for explaining the process.)
The advantage of this method is that there is no chemical residue and the catechins are not leached out with the caffeine (or not as much) as the ethyel acetate method. The taste is also not affected. Most high-end tea purveyors use this method. Harney and Sons has an impressive collection you can order online.
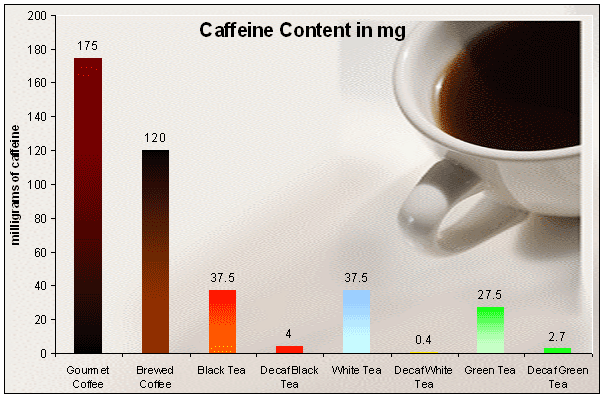
Although tea has less caffeine than coffee, if you don’t want the jingle jangle, try decaf. Just don’t fall for the web talk about doing it yourself.